Product information
Properties of Urocin®
Active ingredient: Mitomycin
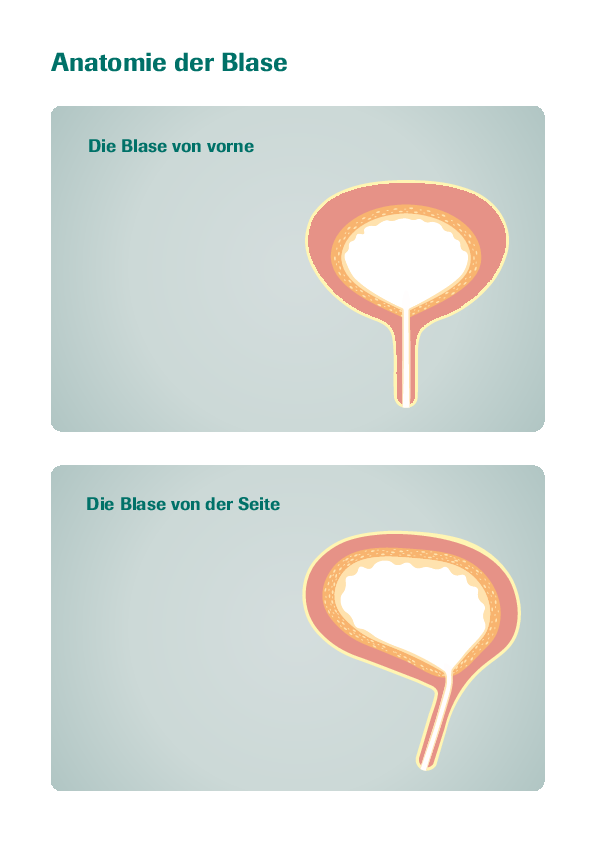
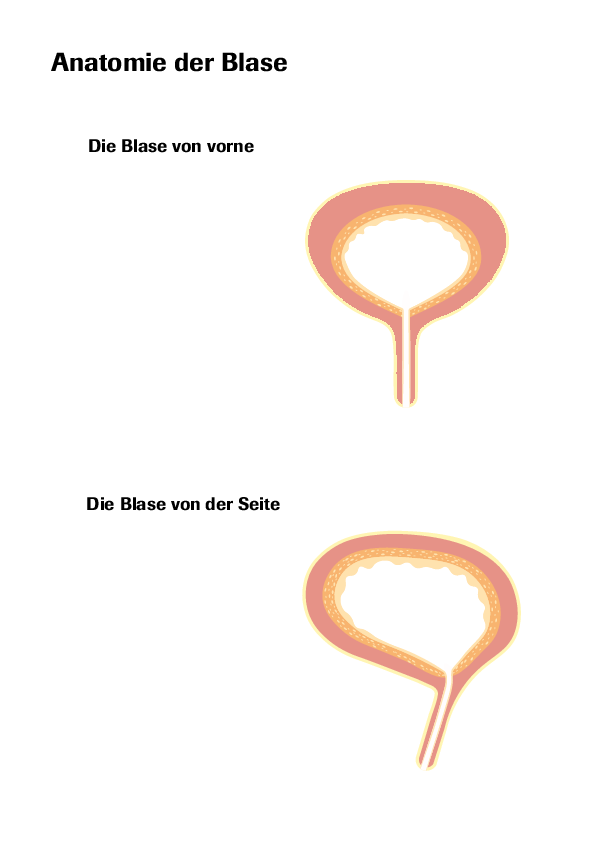
Indication: Urocin® is intended for intravesical use (inside the bladder) to prevent recurrence of superficial bladder cancer following transurethral resection (removal of tissue through the urethra).
Dosage, type and duration of use: Mitomycin should only be used by doctors who have experience with this treatment. It is important to make sure that the treatment is necessary. There are various treatment regimens for mitomycin, which differ in terms of the dosage used, the frequency of application and the duration of therapy.
Specialist group information
Click here to go to the specialist group login. You can see more detailed information after logging in.
Pack sizes
Pack sizes of Urocin®
Urocin® is available in 20 mg and 40 mg pack sizes:
Urocin® 20 mg

| PZN (pharmaceutical identification no | Pack size | Standard size |
|---|---|---|
| 06980531 | 1 vial + bladder instillation system (N1) | N1 |
| 06980548 | 4 vials + bladder instillation system | |
| 06980554 | 6 vials + bladder instillation system (N2) | N2 |
Urocin® 40 mg

| PZN (pharmaceutical identification no.) | Pack size | Standard size |
|---|---|---|
| 14290964 | 1 vial + bladder instillation system (N1) | N1 |
| 14290970 | 4 vials + bladder instillation system | |
| 14290987 | 6 vials + bladder instillation system (N2) | N2 |
Downloads
Free material for download
With just one click you can download the Usage and Technical Information as well as other service material and our patient film with valuable information: